At CoreDux®, we approach every challenge with Trust, Mastery and Commitment. We deliver smart solutions through close collaboration. In CoreDux Challenges, we Share our insights, Solve complex technical problems and Sustain long-term performance for our customers and other stakeholders. These are real stories of co-creation, precision and impact.
Hydrogen Embrittlement: A Hidden Threat to Stainless Steel Hoses
Understanding risks and safeguards in high-pressure hydrogen systems
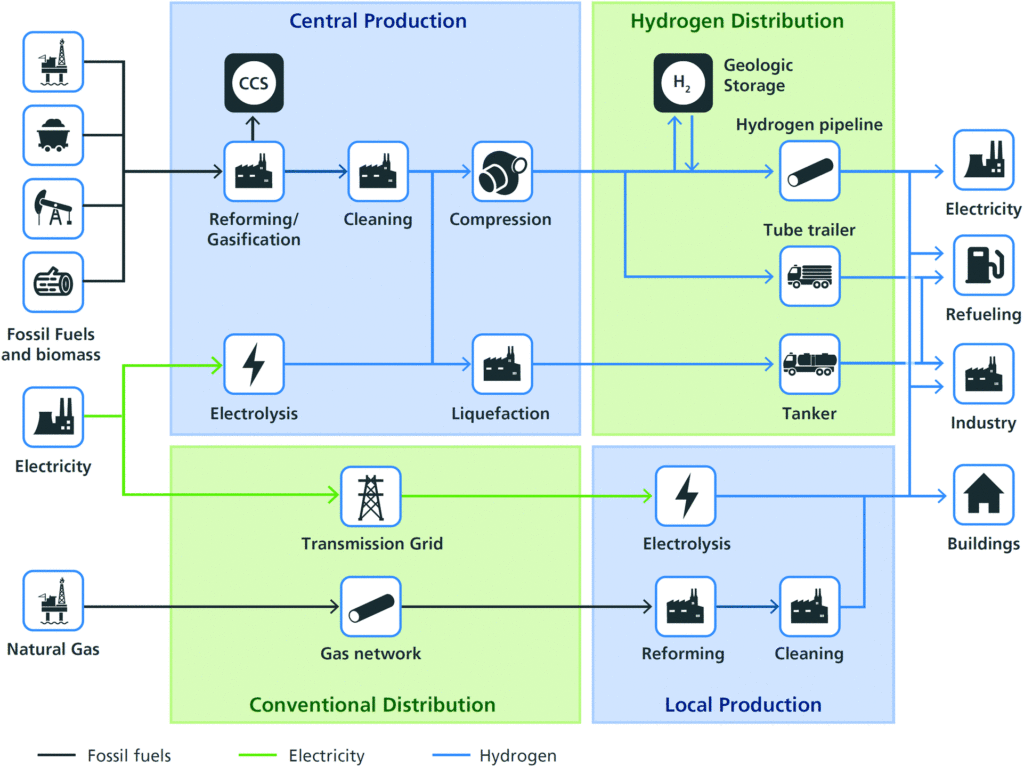
Hydrogen is playing a key role in the shift to clean energy, but working with it comes with serious challenges. One of the biggest threats is hydrogen embrittlement (HE) – a failure mechanism that weakens metals from the inside, leading to unexpected breakdowns in critical systems. Unlike visible wear and tear, HE happens on a microscopic level, making it a silent but dangerous issue for
industries relying on high-pressure hydrogen transport and storage.
Austenitic stainless steel hoses and pipes are widely used in hydrogen systems due to their corrosion resistance and durability. However, even these materials are not immune to embrittlement. Understanding how hydrogen interacts with metal, what increases the risk, and how to prevent failures is essential for engineers, manufacturers, and operators handling hydrogen infrastructure. In this article, we’ll explore the causes, mechanisms, and mitigation strategies for hydrogen embrittlement in austenitic stainless steel hoses, with the aid of case studies from various industries.
How hydrogen embrittlement happens
Hydrogen embrittlement is not an immediate failure; it’s a slow process that weakens metal over time. The issue arises when hydrogen atoms diffuse into the metal structure, reducing ductility and making the material more brittle. The process occurs in three main stages:
- Hydrogen absorption: Hydrogen enters the metal through multiple pathways, such as, high-pressure hydrogen gas exposure in pipelines and storage systems, electrochemical reactions during service, especially in corrosive environments or through manufacturing processes like welding, pickling, and electroplating, which can introduce hydrogen into the material.
- Diffusion and trapping: Once inside, hydrogen atoms move through the metal’s atomic lattice, accumulating at weak points such as grain boundaries, dislocations, and micro-cracks. Over time, these trapped atoms recombine into hydrogen molecules, creating internal pressure within the metal structure.
- Crack initiation and propagation: As hydrogen concentration increases, the material becomes brittle. Micro-cracks begin to form at stress points—such as welds, bends, or areas with residual stress. Under operational loads, these cracks grow and spread, eventually leading to catastrophic failure.
What increases the risk of hydrogen embrittlement?
Not all metals respond to hydrogen exposure in the same way. Several factors determine how susceptible austenitic stainless steel hoses and other metal components are to embrittlement:
Material microstructure: High-strength steels and some nickel alloys are highly vulnerable. Austenitic stainless steels, such as 316L, offer better resistance due to their high solubility and low hydrogen diffusivity, but they are not completely immune.
Hydrogen concentration: The higher the hydrogen pressure, the greater the embrittlement risk. Certain impurities like H₂S accelerate embrittlement.
Stress levels: Areas of high stress, such as welds, bends, and fittings, are particularly vulnerable. This is especially the case for hydroformed hoses, where forming inherently increases local cold deformation and residual stress at the corrugation peaks. Both applied and residual stresses can make hydrogen embrittlement worse. To mitigate this, CoreDux applies special care to optimize corrugation shapes and forming processes, using dedicated designs specifically developed to reduce residual stresses.
Temperature effects: Hydrogen embrittlement is most severe around 80-100°C. Interestingly, metals tend to be less susceptible at temperatures above 150°C, where hydrogen diffusion is faster, but less damaging.
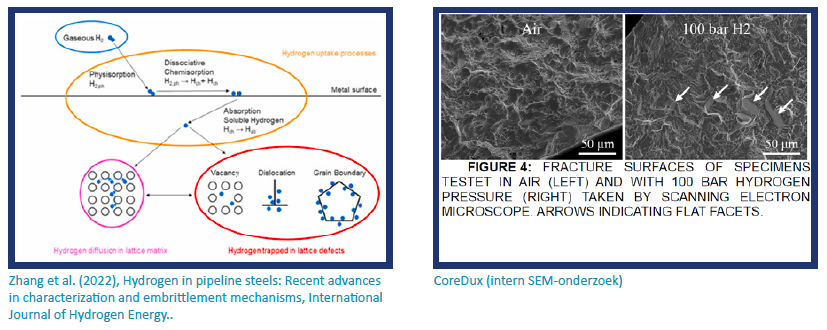
Real-world case studies
Hydrogen embrittlement has been responsible for major failures across different industries. These case studies highlight the risks and how companies have responded.
Case study 1: Hydrogen Pipelines in the Energy Sector
A pipeline carrying high-pressure hydrogen gas suffered repeated failures. Upon investigation, engineers discovered that hydrogen had diffused into the steel, forming micro-cracks at grain boundaries. The embrittlement caused unexpected shutdowns and maintenance costs.
Mitigation: The company switched to lower-strength steels and applied protective coatings to slow hydrogen diffusion.
Case study 2: Space shuttle components
In the space industry, components used in high-pressure hydrogen systems – such as those in the Space Shuttle Main Engine (SSME) – began showing signs of embrittlement. This posed a major risk, as failure mid-mission would be catastrophic.
Mitigation: Engineers redesigned components to minimize hydrogen exposure and selected materials with higher resistance to embrittlement.
Case study 3: Aircraft structural components
Aircraft components made from high-strength alloys developed hydrogen embrittlement during manufacturing. The issue was traced back to processes like pickling and electroplating, which unintentionally introduced hydrogen into the metal.
Mitigation: The company introduced post-processing heat treatments to remove hydrogen before the components were put into service.
Case study 4: Semiconductor manufacturing equipment
In semiconductor fabrication, hydrogen embrittlement affected materials used in manufacturing equipment. The problem was linked to exposure during chemical vapor deposition (CVD), which led to material degradation.
Mitigation: Engineers selected hydrogen-resistant materials and implemented tighter controls on hydrogen exposure in production.
How to prevent hydrogen embrittlement in hoses and pipes
Preventing hydrogen embrittlement requires more than just awareness – it demands strategic choices in materials, manufacturing processes, and design. The first step is selecting the right materials. Austenitic stainless steels with high nickel content are naturally more resistant to hydrogen embrittlement, while high-strength steels and certain nickel alloys are particularly vulnerable. In applications where hydrogen exposure is unavoidable, using metals with lower diffusivity and higher solubility for hydrogen can reduce the risk of failure.
Manufacturing processes also play a critical role. Hydrogen can enter the metal during welding, electroplating, and pickling, which means strict controls are needed during fabrication. Using dry welding rods, maintaining a moisture-free environment, and applying post-processing heat treatments can help remove any residual hydrogen trapped within the metal. These steps prevent embrittlement from starting before the component even enters service.
Once a system is in operation, environmental controls become the next line of defense. Protective coatings or barriers can be applied to limit hydrogen absorption. In corrosive environments, cathodic protection is often used to reduce hydrogen uptake, particularly in underground pipelines or subsea applications where hydrogen exposure from corrosion reactions is a major concern. Even the best materials and manufacturing controls won’t be enough if the design itself creates weak points that accelerate embrittlement. Stress concentrations, such as sharp corners, tight bends, and over-tightened fasteners, become prime locations for crack initiation. Optimizing component geometry, using proper joint structures, and minimizing residual stresses during installation and forming—particularly in flexible hoses where the forming process can significantly
increase residual stress and reduce resistance to embrittlement—can significantly extend the lifespan of metal hoses and pipelines used in hydrogen systems.
Conclusion: controlling the risk
Hydrogen embrittlement is a real and often underestimated risk in hydrogen transport and storage. It weakens metal components at the microscopic level, making failures sudden and unpredictable.
However, it is not an unavoidable consequence of working with hydrogen. By selecting the right materials, controlling hydrogen exposure during manufacturing, and designing systems that minimize stress points, companies can significantly reduce the risk of embrittlement-related failures.
As hydrogen infrastructure continues to grow, industries that take these precautions now will avoid costly failures in the future. With the right approach, hydrogen can be transported and stored safely, ensuring its place as a key component of the clean energy transition.
In short: don’t let hydrogen embrittlement catch you off guard! It may be a silent threat, but one you can control. It’s all about the right design, materials and manufacturing approach. Are you facing an HE challenge, and would you like to discuss your options? Reach out to CoreDux today to talk with one of our engineers specializing in hydrogen embrittlement. They’re here to help you choose what works best.






